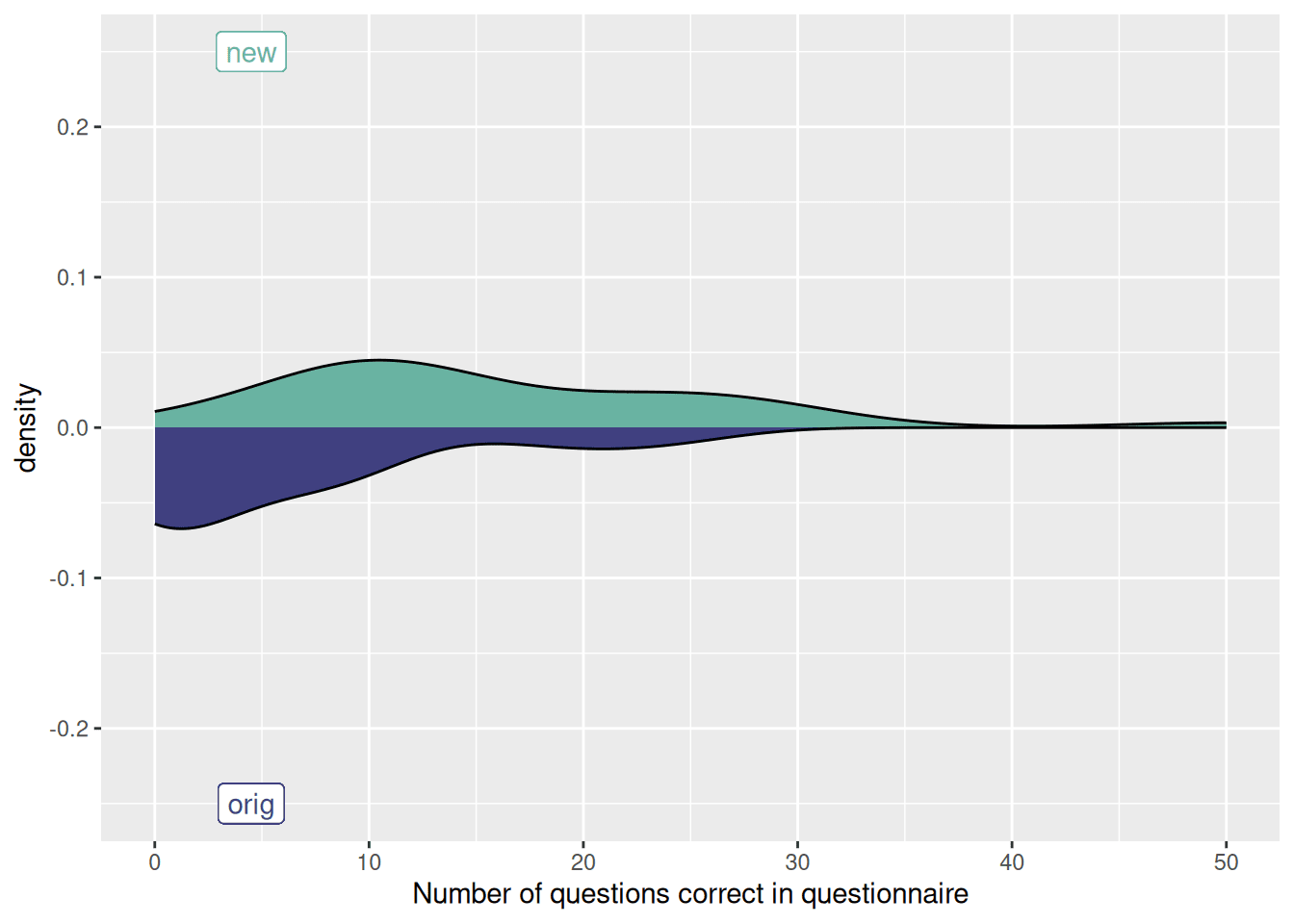
Improving Harmony's PDF extraction with user testing
Since we built Harmony, a common complaint has been that it frequently identifies the wrong questions in PDFs. The original algorithm for finding questions in PDFs was a mixture of rule based heuristics and some hand coded logic to look for e.g. lines in the document which begin with numbers. This was very fragile and worked fine on short questionnaires such as the GAD-7, but failed on larger documents.
We decided to run a competition with our partner DOXA AI where members of the public could train their own model to extract questions from PDFs.
Harmony at GenAI and LLMs night at Google London
Harmony at GenAI and LLMs night at Google London on 10 December 2024 Above: video of the AICamp meetup in London on 10 December 2024. Harmony starts at 40:00 - the first talk is by Connor Leahy of Conjecture
We have presented the AI tool Harmony at the GenAI and LLMs night at Google London on 10th December organised by AI Camp at Google Cloud Startup Hub.
AI Camp and Google hosted two deep dive tech talks on AI, GenAI, LLMs and machine learning, with food/drink, networking with speakers and fellow developers.
Clinical trial and research data harmonisation principles
Clinical trial and research data harmonisation principles In the realm of healthcare, clinical trials serve as the bedrock of evidence-based medicine, guiding decisions that affect patient care and public health policies. However, the effectiveness of these trials hinges on the quality and compatibility of the data they generate. The harmonisation of clinical research data emerges as a pivotal endeavour in ensuring the integrity and interpretability of trial outcomes. In this blog post, we delve into the principles of clinical data harmonisation, exploring its significance in clinical trials and elucidating the strategies.
Data Harmonisation in Real Estate
Overview In this era of digital business, ‘data’ is considered priceless wealth. It’s an important resource which provides businesses with the necessary insights, visions, and suggestions to take on new approaches, continually innovate, and make more informed decisions which benefits everyone in the mix – in case of real estate, from the property owners, landlords, and tenants to real estate agencies, agents, stakeholders, and what have you.
Data harmonisation refers to a process where data is selected from a variety of sources, such as internal operations and external interactions as well as day to day business activities, where the relevant information is synthesised to make it qualitative and easily connect between the different sources.
International cancer data harmonisation in 2024
International cancer data harmonisation – The state of 2024
If we look at the last decade alone, biomedical sciences have changed a lot. The data they must gather on a daily basis has also evolved to be vaster, more natively web-based, more interdependent, and more distributed.
It’s a transformation that has fundamentally diversified not only the actors responsible for conducting analyses but also the types of data accessed.
In order to find the answers to some incredibly complex scientific questions posed by the entire range of stakeholders in healthcare, we need to have specific systems and standards implemented in order to address the challenge of using data that is both unified and meaningful.
Master Data Harmonisation: Key to Business Value
Unlocking Business Value through Master Data Harmonisation “The world is one big data problem.” – Andrew McAfee, Co-director of the MIT Initiative on the Digital Economy. Indeed, data can turn into a significant problem when organizations don’t know how to effectively manage and use it.
Today, businesses deal with a lot of data. If they don’t handle this data well, it can become too much to manage. This can cause businesses to miss chances, work less efficiently, and make bad decisions.
Pharmaceutical and R&D Data Harmonisation: Tools, Standards, and Processes
Overview The pharmaceutical industry is mired in regulatory compliance requirements, which means businesses need to have well-established pharmaceutical R&D data harmonisation standards. Any omission, inaccuracies, inconsistencies or errors in data recording and reporting can carry a lot of risk, which means pharmaceutical R&D data harmonisation companies must be able to provide a complete audit trail of each step within every process – from the development of drugs or products to bringing them to the market to tracking them throughout their lifespan.
Item harmonisation
Conquer the Chaos of Questionnaires: Item harmonisation made easy Researchers in psychology and social sciences frequently face a challenge: comparing data from different questionnaires. For example, they may be combining cohort studies or longitudinal studies or pooling data for cross cohort research. Imagine trying to compare responses to “I often feel anxious” with “Feeling nervous, anxious or afraid” – even though they seem similar, they might not capture the same level of anxiety.
Data Harmonisation in Education
Data Harmonisation in Education: Overview The term ‘harmonisation’ has often been used in different contexts – for example, to describe similar phenomena, such as collaboration, coherence, alignment, integration, partnership, etc. However, we might argue that these concepts might do nothing more than indicate the extent and scale of integration among different entities when it comes to regional cooperation.
Now, the underlying degree of interaction between all the players involved can run a lot deeper and tighter when we transition from collaboration, partnership, and cooperation to integration, community, harmonisation, and interdependence.
How To Find Matching and Common Items in Questionnaires and Surveys
When researchers take on the task of analysing data from surveys and questionnaires, they often encounter a significant obstacle: finding matching or common items across different sources. This challenge is due to the many different ways questions are asked or formatted. This makes it tough to compare and merge data effectively.
According to Forbes, researchers spend up to 80% of their time just getting data ready for analysis, and a big part of that time goes into harmonising data.